Membrane Proteins
Linear Dichroism of Membrane Proteins
A large proportion of proteins are membrane proteins, and processes occurring in or on membranes are essential in biological organisms. Membrane proteins are also important in the development of new drugs with more than half the drugs on the market acting on membranes or membrane proteins. Linear dichroism spectroscopy (LD) can give important information on the orientation of the protein or peptide relative to the membrane. This can elucidate the mode of binding of proteins to the membrane. In addition to this, changes on ligand binding to receptors can be observed. Another important area where LD can contribute useful information is in membrane protein folding, where one will see a change in the LD signal as the protein or peptide inserts into the membrane and adopts its native fold.
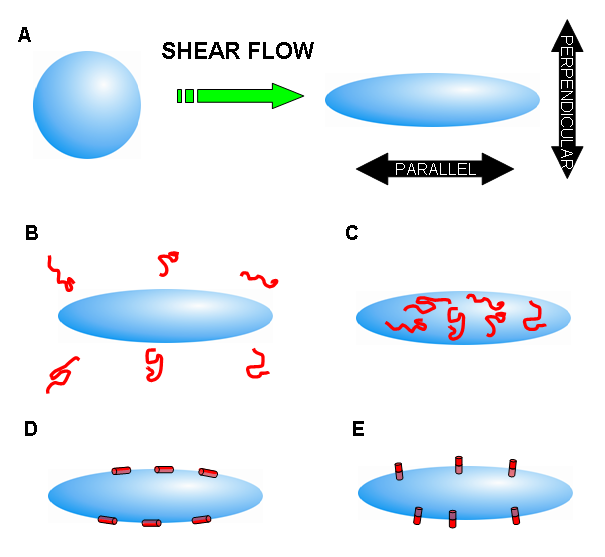
One way of studying membrane proteins in the lab is to make model membranes called liposomes. These spherical aggregates of lipid can be made from the lipids that are found in nature. Membrane proteins will not align by themselves, but the liposomes in which they are inserted can be aligned. The alignment happens because the liposomes deform due to shear flow caused by stirring.
The LD signal will be different depending on how the peptide or protein inserts into the membrane. For example, the signals will be of opposite sign for D and E shown below. More precise orientation angles and subtle changes can also be measured by using a probe in the lipid to give a measurement of the extent to which the liposomes are aligned in the sample.
We are developing high-throughput screening methods for membrane-protein interactions that will be invaluable in the search for new drug leads that act in or on membranes.
We are also collaborating with several groups working on membrane proteins such as the important class of receptor molecules G-protein-coupled receptors (GPCRs).
Publications:
Hicks, M. R., Dafforn, T., Damianoglou, A., Wormell, P., Rodger, A., and Hoffmann, S. V. (2009) Synchrotron radiation linear dichroism spectroscopy of the antibiotic peptide gramicidin in lipid membranes The Analyst 134(8), 1623-1628.
Ennaceur, S. M., Hicks, M. R., Pridmore, C. J., Dafforn, T. R., Rodger, A., and Sanderson, J. M. (2009) Peptide Adsorption to Lipid Bilayers: Slow Rearrangement Processes Revealed by Linear Dichroism Spectroscopy Biophysical journal 96(4), 1399-1407.
Oates, J., Hicks, M., Dafforn, T. R., DiMaio, D., and Dixon, A. M. (2008) In vitro dimerization of the bovine papillomavirus E5 protein transmembrane domain Biochemistry 47(34), 8985-8992.
Hicks, M. R., Damianoglou, A., Rodger, A., and Dafforn, T. R. (2008) Folding and membrane insertion of the pore-forming peptide gramicidin occur as a concerted process J Mol Biol 383(2), 358-366.
Hicks, M. R., Conner, M., Dafforn, T., Knowles, T. J., Ludwig, C., Staddon, S., Overduin, M., Gunther, U. L., Thome, J., Wheatley, M., Poyner, D. R., and Conner, A. C. (2008) Functional and biophysical analysis of the C-terminus of the CGRP-receptor; a family B GPCR Biochemistry 47(32), 8434-8444.
Rajendra, J., Damianoglou, A., Hicks, M., Booth, P., Rodger, P. M., and Rodger, A. (2006) Quantitation of protein orientation in flow-oriented unilamellar liposomes by linear dichroism Chemical Physics 326(1), 210-220.
