Paul Shaw
Under the supervision of Jon Rourke I have been continuing the research into organo-platinum chemistry.
Over the last year I have been oxidizing Pt(II) dicyclometallated complexes of the type C^N^C (2,6-di(4-fluorophenyl)pyridine) with PhICl2. This oxidant adds to the metal in a 2 step process, involving formal addidion of Cl+ to form a 5-coordinate intermediate. With very small ligands (e.g. DMSO) the product that quickly forms even at low temperatures, is where the two chlorides are trans across the metal. This then isomerises to form a cis complex.
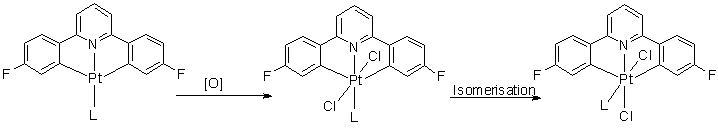
When L is PPh3 the steric bulk inhibits the formation of the 6-coordinate complex, and so the 5-coordinate intermediate is stable (even at room temperature) and can even still be seen 3 hours later.
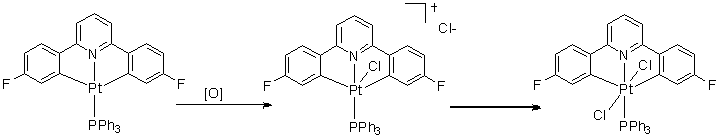
When L is PnBu3 (apart from the formation of the obvious trans product) the butyl chains are long enough to loop back and an agostic complex is formed at low temperatures (-60 °C), where a C-H bond fills the remaining coordination site. Upon warming to around -20 °C this product disappears with the appearence of a new product, thought to be where the C-H bond is now activated.

