Matthew Turner
I am currently in the Third year of my PhD supervised by Nick Hine and co-supervised by Vas Stavros.
my email: m.turner.1@warwick.ac.uk
PhD project
In my PhD I am fortunate to be working with both Nick Hine and Vas Stavros, both of which supervised my previous masters projects (see below). The work I did with Nick in the second miniproject formed the basis of my PhD although with a different test set of molecules and more focus on studying fluorescent properties with help from Vas Stavros.
My PhD primarily regards the use of linear scaling time dependent density functional theory in order to perform calculations with explicitly modelled solvent molecules. Time dependent density functional theory (TDDFT) traditionally scales in computational time with N3 where N is the number of atoms, in that if you double the number of atoms in a system you multiply the time taken to complete a calculation by 8. Fortunately, owing to recent advances in TDDFT the scaling has been reduced to effectively linear1 hence it is now possible to perform TDDFT on significantly larger systems.
Within the PhD I am making use of this to examine the effect of solvents upon the electronic properties of molecules. Previously this has been widely done using implicit solvent models wherein the effect of the solvent is modelled using a polarizable continuum. This is good for calculating the effect of differing dielectric constant but cannot take into account non-electrostatic effects for instance hydrogen bonding or band hybridisation. I will be using explicit solvent models to better understand these non-electrostatic effects. There are two main problems I am attempting to solve with explicit solvent modelling. Firstly, I will attempt better understand solvatochromic shift and hence be able to more accurately predict the colour of molecules in solution, as well as their fluorescent properties. Secondly, I will be using explicit modelling to better understand the excited state energy decay pathways associated with given molecules in solution. The latter will be benchmarked using results obtained from transient electronic absorption spectroscopy.
MSc projects at University of Warwick
In order to complete the MSc section of the MAS CDT integrated PhD two miniprojects must be submitted.
Project 1: Tuning Fluorescent Yields in Maleimide Based Fluorophores
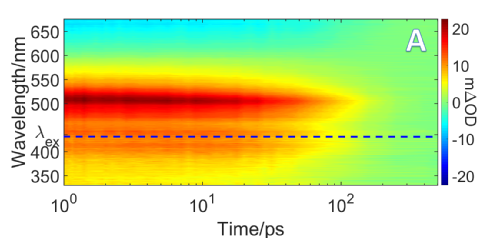
The above figures show transient absorption spectroscopy results for maleimide in cyclohexane. The left figure showing a heatmap of intensity vs wavelength and time delay. The right figure shows the fitted decay assosiated spectrum fitted using the program glotaran.
This project was co-supervised by Vas Stavros and Rachel O'Reilley. The purpose of the project was to investigate the fluorescent properties of tags based on the molecule maleimide. The molecules appeared to have different fluorescent yields when in polar or protic solvents than in a non-polar protic solvent. The reasoning behind this was explored by adding different substituent groups onto the maleimide and examining their excited state properties using transient absorption spectroscopy (TAS).
It was found that protic solvents underwent proton transfer with the oxygen on the maleimide and the energy taken to undergo this effect quenched fluoresence effects. This was attempted to be blocked by an intermolecular hydrogen bond from a substituent group. The TAS results confirmed that this was successful although other effects seemed to be caused by the addition of this substituent group which would have been studied further should there have been more time on the project.
Project 2: Determination of Impurities in Drug Samples through Colour Prediction
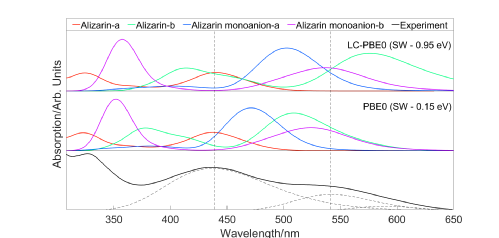
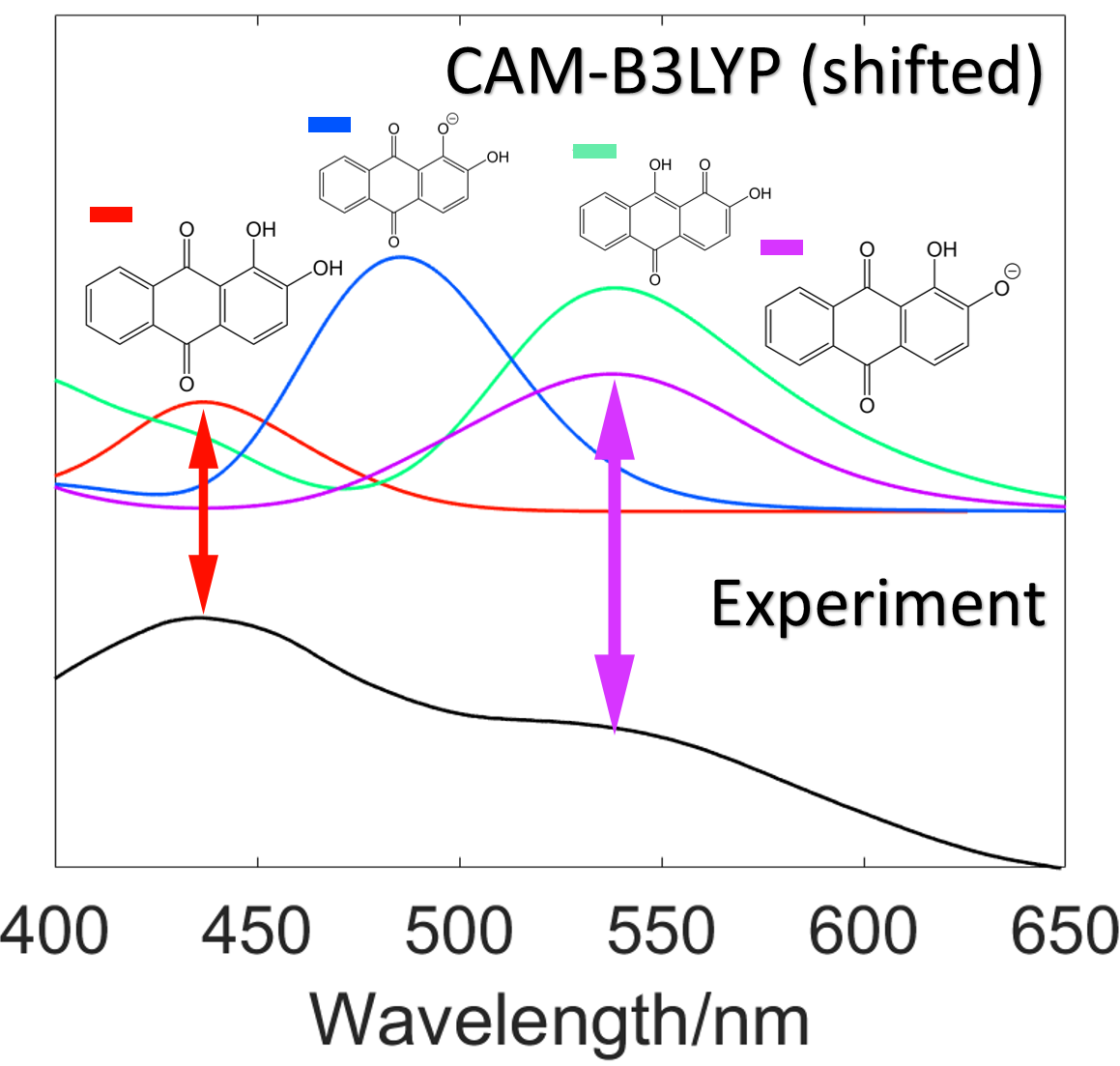
Shown above are results for work on alizarin. The far left image shows alizarin in a box surrounded by methanol as generated by molecular dynamics simulations and the centre image shows a hydrogen bond present in this analysis. The far right image shows predicted UV-Vis spectrum for alizarin using different levels of theory and functionals.
In this project, undertaken with Nick Hine of the physics department, I used time dependant density functional theory (TDDFT) in order to predict the colours of drug impurities. We worked with industry, astrazeneca, in order to decide upon the direction of the project.
The first step was to generate a test set of molecules. It was decided that anthracenes were a good candidate as they exhibit an array of colours and often occur as a side product in drug synthesis. These molecules were analysed in methanol as this, as a polar and protic solvent, allowed us to best test the influence of solvent on the predicted colour of our molecules, an effect known as solvatochromic shift. The molecules were analysed in vaccuu, in an implicit COSMO solvent model, and finally in an explicit solvent model.
Explicit solvent model calculations are generally not used owing to their high computational expenditure. These calculations are important, however, as implicit solvent models don't take into account non-electrostatic effects whereas explicit solvent models do. Fortunately we were able to undergo explicit solvent calculations by using linear scaling density functional theory methods developed by the ONETEP group. Further to this a cheap PBE functional was used and spectral morphing was conducted as orignally shown by Baroni et al. (X. Ge, I. Timrov, S. Binnie, A. Biancardi, A. Calzolari and S. Baroni, The Journal of Physical Chemistry A, 2015, 119, 3816– 3822).
MChem Project at the University of Reading
In my first masters degree I untook a project with David Nutt in which I modelled the infrared spectra of oleic acid derivates using density functional theory. Oleic acid splits into four main derivatives when exposed to UV radiation: nonanal, nonanoic acid, 9-carboxynonanoic acid, and azaliac acid, these were the molecules studied. This project had a more atmospheric chemistry direction as the oleic acid derivates where treated as if in a water droplet, as they would be found in the upper atmosphere.
Other interests
Further to my academic interests I also enjoying playing football as part of a team in Calundon and sailing with my family.
References
1. C.-K.Skylaris, P.D.Haynes, A.A.Mostofiand, M.C.Payne,The Journal of chemical physics, 2005, 122, 084119.

