My Phd
My Phd project was an extension of my second mini-project (for more about my miniprojects click here). The aim was to apply Evanescent Wave Cavity Ring-Down Spectroscopy (EW-CRDS) combined with electrochemical/fluidic techniques to probe surface reactions in both in the biological sciences. I was supervised by Prof Pat Unwin, Prof Julie Macpherson from the Electrochemistry and Interfaces Group , Dr Teresa Pinheiro from the Structural Biology Group , and Dr Stuart Mackenzie from the Chemistry Dept in Cambridge.
My Advisory Committee were Dr Corinne Smith (Biology), Dr Vasilos Stavros (Chemistry) and Dr Tiffany Walsh (Chemistry).
The Importance of Biointerfaces
The cell membrane provides the stage for many essential cellular processes, such as energy transduction. Therefore elucidation of the mechanisms of these processes is critical to understanding the inner workings of the cell. Additionally, the development of technologies such as biosensors, biofuel cells and biocompatible materials relies on this knowledge. Instruments that can study such processes in situ and with good temporal resolution are few in number. The combination of the high spectral and temporal sensitivity of EW-CRDS with the electrochemical/fluidic methods as a delivery method ought to prove invaluable in the study of the kinetics and mechanisms of fast biologically-relevant surface processes.
A little bit about Evanescent Wave Cavity Ring-Down Spectroscopy (EW-CRDS)
Cavity Ring-Down Spectroscopy is an ultra-sensitive absorption technique which was initially developed by the aerospace industry for the characterisation of highly reflective mirrors. The simplest kind of Cavity Ring-Down Spectrometer consists of a light source coupled into a high-finesse optical cavity made up of two highly reflective mirrors as seen in Figure 1.
![[PHOTO]](crds.jpg)
Figure 1: A simple CRDS
The light source enters the cavity through the back of one mirror and is reflected back and forth between the two. On each pass around the cavity the intensity of light transmitted through the back of the other mirror is recorded. Since light is lost due to a small leak out of the back of the mirrors, scattering and the absorption of any species within the cavity the light intensity recorded decreases exponentially with time (see Figure 2).
![[PHOTO]](ring-down.jpg)
Figure 2: A typical ring-down trace
The Ring-Down time is defined as the time in which the trace decreases to e-1 of its initial value. If an extra absorbing species is put in the cavity then the ring-down time will decrease. Using the Beer-Lambert law it can be shown that the absorption coefficient of this species can be calculated using the formula
![[PHOTO]](absorbance.jpg)
where τ0 is the ring-down time of the empty cavity.
In EW-CRDS the cavity includes a prism. In our set-up in the lab the cavity consists of a triangular arrangement of two mirrors and a prism as seen in Figure 3. The light enters the back of one mirror, is reflected off the other before undergoing total internal reflection in the prism and ending up back at the first mirror. Upon total internal reflection in the prism an evanescent wave is formed at the prism/air interface. Anything put in the evanescent wave can absorb and so will contribute to light loss in the cavity and hence reduce the ring-down time. Thus by placing a cell on top of the prism absorbance measurements of samples in the condensed phase can be recorded in the same way as for the simple CRDS described previously.
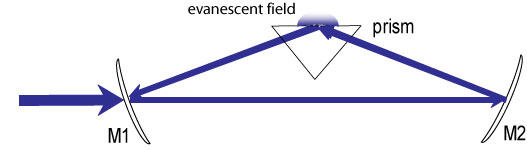
Figure 3: The optical cavity used in EW-CRDS
EW-CRDS and Electrochemistry: A combined approach
EW-CRDS was combined with electrochemistry by placing a working electrode over the evanescent field, a few hundred microns from the surface. The electrode can then be used to deliver analyte to the surface to induce a surface process in a well-defined manner. Since the delivery is exceptionally well controlled, the entire situation can be modelled mathematically to fit the experimental data and infer mechanisms and obtain kinetic rate constants for the induced process. To resolve really fast processes, the electrode can be replaced with an impinging jet to deliver analyte to surface rapidly.
Applications
During my PhD I was involved in a number of projects. The first was a study of the adsorption of [Ru(bpy)3]2+ to polypeptide films. For me, this was a stepping-stone study as it was the first demonstration of an EW-CRDS investigation that didn't involve the silica-water interface. The ability to modify the prism with polyelectrolyte films and subsequently study interactions with these films, provided a platform on which the interaction of molecules with much more complex biologically relevant surfaces could be studied. Subsequent to this, I investigated the possibility of modifying the prism surface with a supported lipid bilayer to mimic the cell membrane. I was successful in creating simple bilayers (i.e. composing of a single type of phospholipid) and monitoring the adsorption of a porphyrin molecule to the resulting bilayer surface. Work is ongoing to create more realistic bilayers containing difference compositions of phospholipids. I was also involved in a study of the interaction of a porphyrin with calf-thymus DNA. Such interactions are important for anti-tumor and anti-viral applications. Finally, I explored the electrochemistry of the redox protein cytochrome c. This was a particularly exciting study as EW-CRDS uniquely has the potential to study the kinetics of such redox reactions of biomolecules in biologically-relevant environments. All of these studies have been published (or are in the process of being published) so see my Publications page for more information.
