Light Emitting Diodes
Introduction
A Light Emitting Diode is a semiconductor device that produces monochromatic incoherent light. The device consists of a p-n junction with a forward bias applied.

Figure 1: A collection of LEDs. (Photo used under creative commons from 'Q-branch' http://www.instructables.com/file/FV00U508ZPEP27TMES/)
Device Operation
When no bias voltage is applied to the diode containing the p-n junction, there is a potential difference across the junction that prevents the diffusion of electrons and holes across the barrier. A bias voltage can be applied using a battery. Connecting the positive terminal to the p side of the junction applies a forward bias that decreases the potential difference across the junction, increasing the diffusion rate of electrons and holes and allowing a current to flow, as shown in Figure 2. Connecting the terminals the other way round leads to negative bias, which increases junction potential difference and prevents current flow.
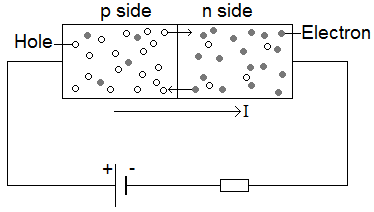
Figure 2: A diode with positive bias applied, leading to current flow and the ejection of minority carriers into majority carrier regions (electrons into the p-side and hole into the n-side)
The application of a forward bias to the diode causes electrons to flow into the p region from the n region and holes to flow into the n region from the p region. When they enter the new region, the carriers constitute minority carriers amongst majority carrier types, which rapidly leads to recombination. In the direct recombination process, an electron from the conduction band falls into a hole in the valence band whilst conserving energy by emitting a photon, as shown in Figure 3. LEDs are hence made from direct bandgap semiconductors where the majority of recombinations do not involve phonons. Excess carriers come into thermal equilibrium before recombination, meaning that most emitted photons have energy equivalent to the bandgap energy. The bandgap of the material therefore determines the colour of the emitted light and this can be modified by changing the semiconductor material.
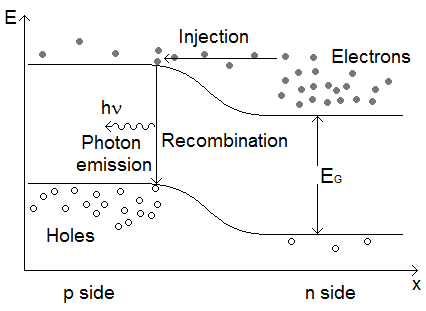
Figure 3: Diagram showing energy levels of LED against physical distance. The recombination of electrons and holes leads to the emission of photons with energy equivalent to the material bandgap.
References
1. J. R. Hook and H. E. Hall, Solid State Physics, 2nd Ed, Wiley, 1991.
2. P. A. Tipler and G. Mosca, Physics for Scientists and Engineers, 5th Ed, W. H. Freeman and Co., 2004
