Daniela Lobo
About scientists
Science has always fascinated me. I believe my penchant for this field stems from my perception of what it is that scientists do: they lead to an ever-increasing knowledge of the world in a constant attempt to make sense of it all. "Observe. Explore. Create." We build models that can explain and predict the behavior of small parts of the world, being that a bacteria, the beating of a heart or a thunderstorm. But often, we fail and get frustrated, confused and we think it's going to work but it doesn't. And so we go for a walk (yes, scientists walk a lot!). To think, to observe. To seek for inspiration and meaning and answers, waiting for some "Eureka!" as we stare at a tree or a bird (yes, scientists stare at weird things a lot!). But often, nothing happens. We explore alternatives, previous mistakes, new ways to get there. And we hope we'll be able to accept that the "small part of the world" we can perceive is so little compared to what’s out there. We hope that will only keep us going. We're interested not only on chemistry, biology and physics. We're interested on the moment where everything is too confusing to understand but too interesting to quit. Not the 'Eureka!' moment (it's overrated anyway), but instead the 'Isn't that funny?' moment. And when that moment starts tinkling in the back of our head, we create.
About me
My research aims to build an on-line detector for biopharmaceutical activity. That may be done by measuring binding of active ingredients to antibodies which are covalently attached to engineering modified M13 bacteriophages. By doing so, we hope to be able to explore new methods for quality by design, confirming molecular identities, ensuring it’s consistently formulated with a well-defined oligomeric state and folded in the correct molecular structure. It’s an interdisciplinary project with bits of physics, chemistry, engineering and biosciences. Recently, I’ve been designing fluorescent phages able to detect variations on blood flow in small capillaries, as a way to, for example, determine how well angiogenesis inhibitors therapy works on cancer patients.
Academic History
September, 2013 - September, 2016: PhD, University of Warwick, Department of Chemistry, Biophysical chemistry group
- 'Functional on-line testing of biopharmaceuticals'
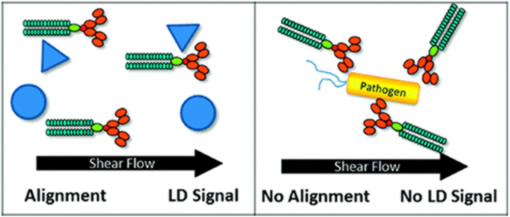
R Pacheco-Gomez, J Kraemer, S Stokoe, HJ England, CW Penn, E Stanley, A Rodger, J Ward, MR Hicks, TR Dafforn (2012) Detection of Pathogenic Bacteria Using a Homogeneous Immunoassay Based on Shear Alignment of Virus Particles and Linear Dichroism. Analytical Chemistry 84 91-97.
Supervisors: Prof. Alison Rodger, Dr. James Covington, Prof. Tim Dafforn, Dr. Matthew Hicks.
September, 2008 - July, 2013: University of Coimbra, School of Pharmacy (Portugal)
- Master's Degree on Pharmaceutical Sciences
Previous Experiences
May, 2013 - August 2013: UCL, School of Pharmacy, Department of Pharmaceutics (UK)
- Electrospinning of PEO/SA/Drug 3D nanofibers as a new drug delivery system.
June, 2012 - August, 2012: Medical University of Warsaw, Department of Immunology and Molecular Genetics (Poland)
- Immunophenotyping using flow cytometry. Click-iT© Detection Assays. Click-iT© EdU Cell Proliferation Assays.
September, 2011 - June, 2012: Histocompatibility Centre, Department of Molecular Biology (Portugal)
- Characterization of the immune response to hepatitis C virus (HCV) in pacients infected with HCV and patients co-infected with HIV-1/HCV, during therapy with pegylated interferon-α and ribavirin. Association of response mediated by CD8+ T lymphocytes and dendritic cells with treatment success.
- Training on preparing blood/tissue samples and cell cultures. Differential diagnosis of viral and self-immune diseases (anti-SSA/Ro antibodies). Serological diagnosis of syphilis. Culture isolation of M. tuberculosis. Coombs test. ELISA. PCR. Blotting methods. Fluorescence microscopy.

Contact Details
Address:
Chemistry Department, B 608
Email: D.P.C.A.Lobo@warwick.ac.uk
