How to build a better railway - in (almost) every cell in your body -
New research from the University of Warwick shows how a microscopic ‘railway’ system in our cells can optimise its structure to better suit bodies’ needs.
The work was conducted by Professor Robert Cross, director of the centre for mechanochemical cell biology at Warwick Medical School and leader of the Cross lab. His team based at Warwick Medical School has been looking at how the microtubule ‘railway tracks’ inside cells are built.
Almost every cell in our bodies contains a ‘railway’ network, a system of tiny tracks called microtubules that link important destinations inside the cell. Professor Cross’ team found the system of microtubule rails inside cells can adjust its own stability depending on whether it is being used or not.
Prof Cross said: “The microtubule tracks of the cellular railway are almost unimaginably small - just 25 nanometres across (a nanometre being a millionth of a millimetre).The railway is just as crucial to a well-run cell as a full-size railway is to a well-run country. For cells and for countries the problem is very much the same - how to run a better railway?”
"Imagine if the tracks of a real railway were able to ask themselves, ‘am I useful?’ To find out, they would check how often a railway engine passed along them.
“It turns out that the microtubule railway tracks inside cells can do exactly that - they check whether or not they are in contact with tiny railway engines (called kinesins). If they are, then they remain stably in place. If they are not, they disassemble themselves. We think this allows the sections of microtubule rail to be recycled to build new and more useful rails elsewhere in the cell.”
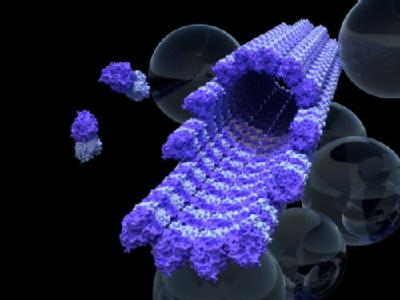
The paper, 'Kinesin expands and stabilizes the GDP-microtubule lattice' published (12 March 2018) in Nature Nanotechnology, shows that when the kinesin railway engines contact their microtubule rails, they subtly change their structure, producing a very slight lengthening that stabilises the rail.
Using a custom built microscope, the Warwick Open Source Microscope, the researchers who are also based at Warwick Systems Biology Centre and Mathematics Institute, University of Warwick, detected a 1.6% increase in the length of microtubules attached to kinesins, with a 200 times increase in their lifetime.
By revealing how microtubules are stabilised and destabilised, the team hope to throw new light on the workings of a number of human diseases (for example Alzheimer’s), which is linked to abnormalities in microtubule function.
They are hopeful also that their work may ultimately lead to improved cancer therapy because the railway is so vital (for example for cell division), as its microtubule tracks are a key target for cancer drugs such as Taxol. Exactly how Taxol stabilises microtubules in cells remains poorly understood.
Professor Cross added: "Our new work shows that the kinesin railway engines stabilise microtubules in a Taxol-like way. We need to understand as much as we can about how microtubules can be stabilised and destabilised, to pave and illuminate the road to improved therapies."
The research was funded by the Biotechnology and Biological Sciences Research Council via the Systems Biology Doctoral Training Centre, University of Warwick; and the Wellcome Trust.
12 March 2018
Image caption: a single microtubule “railway track” surrounded by bubbles of ‘cargo’ held inside cells.
Notes to Editors
Kinesin expands and stabilizes the GDP-microtubule lattice published in Nature Nanotechnology
Authors Daniel R. Peet: Centre for Mechanochemical Cell Biology, Warwick Medical School, Coventry, UK;Warwick Systems Biology Centre, University of Warwick, Coventry, UK
Nigel J. Burroughs: Warwick Systems Biology Centre, University of Warwick, Coventry, UK; Mathematics Institute, University of Warwick,
Robert A. Cross Centre for Mechanochemical Cell Biology, Warwick Medical School, Coventry, UK
This research was funded by the Biotechnology and Biological Sciences Research Council (grant number BB-G530233–1) via the Systems Biology Doctoral Training Centre, University of Warwick; and the Wellcome Trust (grant number 103895/Z/14/Z).
The DOI for this paper is 10.1038/s41565-018-0084-4
For further details contact For further details contact Nicola Jones, Media Relations Manager University of Warwick 07920531221 or N.Jones.1@warwick.ac.uk
